Biomass gasification should be thought of as a refining process that takes a crude feedstock (in our case, woody biomass such as wood chips and nut shells), and processes it into a clean burning gas that is compatible with internal combustion engines. This process consumes a portion of the energetic content of the feedstock in order to achieve this transformation.
Biomass gasification involves sending the woody biomass feedstock through the following five processes:
The resulting gas, known as syngas or producer gas, is a mixture of carbon monoxide and hydrogen, along with nitrogen from the atmospheric air used in the reaction. The other product of this process is charcoal, which also contains the ash content of the biomass. This charcoal byproduct is also known as char-ash. This char-ash is referred to as biochar when it is used as a soil amendment, and known as biocarbon when used for industrial purposes.
The following diagram shows the flow of materials, starting with biomass and ending with gas and charcoal, arranged along the central column of the diagram, while the horizontal bars indicate the process that transforms the materials at each step of the way. Each of the descriptions below refer to this diagram.
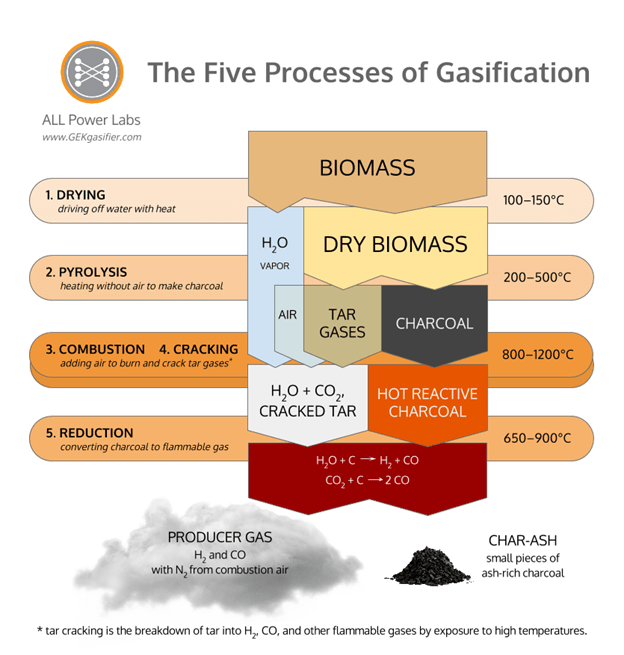
The drying process involves applying enough heat to biomass to drive off all the water. This is an endothermic process that happens in the temperature range of 100-150˚C. The resulting materials emerging from this process are water vapor and dry biomass.
Pyrolysis involves applying heat to the dry biomass to cause it to smoke and turn to charcoal. This is also an endothermic process. The charring process is simply the separation of the volatile compounds (the smoke) from the fixed carbon (charcoal) content of the biomass through the application of heat.
Since drying and pyrolysis are both endothermic processes, a source of heat is needed to drive both of these processes. The combustion of the smoke released during pyrolysis provides the required heat. During the combustion stage, air is introduced into the gasifier and mixed with the smoke so that it burns extremely hot, producing temperatures in excess of 800˚C. Water vapor and carbon dioxide are produced during combustion. The high temperatures also carry out tar cracking.
Woody biomass consists of roughly 80% volatile compounds (by mass) that come off of the biomass as smoke, and 20% fixed carbon which remains as charcoal, with about 1% of ash somewhere between the two. These volatile pyrolysis gasses are known as tar gasses because they condense into tar. These gasses are undesirable because they are acidic, and their condensates are harmful to the moving parts of internal combustion engines. The tar cracking process, which occurs concurrently with combustion, occurs when the heavy organic molecules of these tar gasses break into lighter non-condensing combustible gasses due to exposure to extremely high temperatures. Roughly half of the combustible molecules in syngas come from the cracking of tar.
The water vapor and carbon dioxide resulting from the combustion of volatile pyrolysis gasses are combustion waste products, but they can be converted into combustible gasses by exposing them to reduction reactions. The reduction reactions are shown in the graphic below:
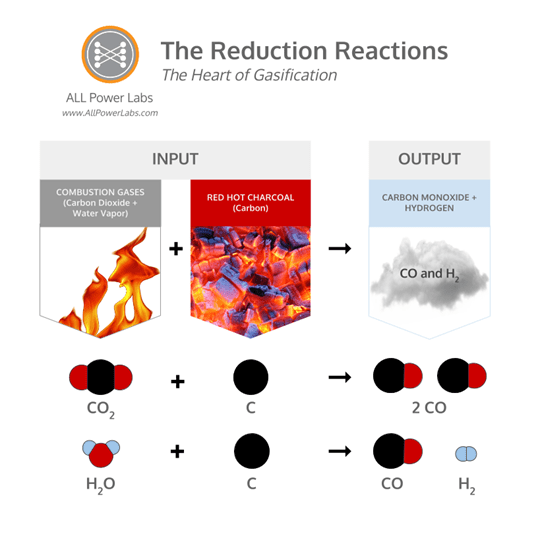
When charcoal is heated to very high temperatures, the carbon content of the charcoal becomes very reactive, and exhibits a very strong affinity for oxygen, enabling it to reduce (the opposite of oxidize; in this context, to ‘reduce’ is to reverse oxidation) oxidized substances such as carbon dioxide and water vapor. As the combustion products from the combustion stage are percolated through the hot charcoal, these reduction reactions convert carbon dioxide and water vapor into carbon monoxide and hydrogen, while consuming carbon from the charcoal to do so. This process produces roughly half of the combustible molecules in syngas.
In the process of reduction, the charcoal chips are perforated at the molecular scale as carbon atoms are individually removed from the surface of the charcoal. This makes the resulting charcoal quasi-activated, which is a desirable quality for filtration, and may potentially have benefits for the charcoal when used as biochar, due to the apparent beneficial influence of increased porosity on ammonia, methane, and N2O emissions abatement.
In the course of gasification, wood chips turn into charcoal chips, and these charcoal chips give up their carbon content during the reduction process to produce carbon monoxide. This loss of carbon causes the charcoal chips to shrink. Eventually, the shrunken charcoal chips pack too densely to permit the rate of gas percolation needed to feed the engine drawing the gas from the gasifier. In order to restore the rate of gas production, the reactor purges the shrunken chips of charcoal, which are then pushed out of the reactor as char-ash. This byproduct of gasification is one of the two material outputs of gasification; the other output is the syngas.
In the process of reduction, the charcoal chips are perforated at the molecular scale as carbon atoms are individually removed from the surface of the charcoal. This makes the resulting charcoal quasi-activated, which is a desirable quality for filtration, and may potentially have benefits for the charcoal when used as biochar.
Depending on what product is desired (gas or charcoal) the gasifier may be operated to produce more of the desired product by regulating how long the charcoal is exposed to reduction reactions before being purged